Introduction to Brallobarbital
Brallobarbital, a barbiturate developed in the 1920s, emerged from a period marked by significant advances in medicinal chemistry and pharmacology. During this era, the search for effective sedatives and hypnotics was a primary focus within pharmaceutical circles due to the rising prevalence of sleep disorders and the necessity for improved therapeutic interventions. Barbiturates, synthesized initially in the late 19th century, were gaining attention as a promising class of compounds capable of producing sedation and sleep induction, positioning them as valuable tools in the treatment of insomnia.
The creation of brallobarbital was pioneered amidst this backdrop of scientific inquiry and clinical need. Researchers were driven by the desire to craft a compound that could safely and efficiently facilitate sleep for individuals experiencing chronic insomnia. Brallobarbital’s development leveraged the barbiturate backbone structure, known for its central nervous system depressant properties. By manipulating the chemical structure, scientists aimed to enhance its therapeutic efficacy while mitigating undesirable side effects—a common challenge with earlier barbiturates.
In clinical practice, brallobarbital was initially esteemed for its ability to induce a calm, hypnotic state, rendering it a preferred choice for patients struggling with sleep disturbances. Its introduction into the pharmaceutical market was timely, coinciding with the heightened demand for reliable sedatives. Physicians of the time appreciated brallobarbital’s potential to alleviate insomnia symptoms and improve sleep quality, thus contributing to better overall patient health and wellness.
The historical context surrounding the evolution of brallobarbital is indicative of a broader movement in medicine seeking to refine sedative agents. The exploration and subsequent incorporation of barbiturates like brallobarbital represented a pivotal phase in the management of insomnia, laying the groundwork for future pharmacological advancements.
Pharmacological Properties of Brallobarbital
Brallobarbital, like other barbiturates developed in the 1920s, operates primarily by depressing the central nervous system (CNS). Its primary mechanism of action involves enhancing the effect of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter in the brain. By binding to GABA receptors, brallobarbital prolongs the opening of chloride channels, leading to hyperpolarization of neuronal membranes. This hyperpolarization results in reduced neuronal excitability, thereby inducing sedation and hypnosis, making brallobarbital effective for treating insomnia.
The typical dosage of brallobarbital for medical use generally ranged from 50 to 200 mg, depending on the severity of the individual’s condition and their response to the drug. Upon administration, brallobarbital exhibits a relatively rapid onset of action, typically within 15 to 30 minutes, which was a desirable trait for patients needing immediate relief from insomnia or anxiety. The duration of its hypnotic effect commonly lasts between 6 to 8 hours, which aligns well with the human sleep cycle, providing a full night’s rest without residual drowsiness in the morning for most patients.
Brallobarbital is metabolized primarily in the liver through pathways involving hydroxylation and conjugation with glucuronic acid, resulting in water-soluble metabolites that are then excreted via the kidneys. This metabolic process ensures that brallobarbital is efficiently cleared from the body, reducing the risk of accumulation and potential toxicity with repeated dosing.
When compared to other barbiturates of its time such as phenobarbital or pentobarbital, brallobarbital displayed a similar profile in terms of sedative efficacy but with a somewhat shorter duration of action. This characteristic made it particularly useful for evening dosing to address sleep latency issues without causing next-day sedation. Its pharmacological properties positioned brallobarbital as a valuable option among the various barbiturates used for insomnia treatment in the early 20th century.
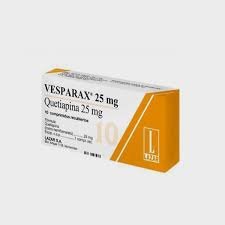
Vesparax: The Combination Product
Vesparax was a notably sophisticated pharmaceutical formulation developed to address chronic insomnia, combining brallobarbital (50 mg), secobarbital (150 mg), and hydroxyzine (50 mg). The rationale behind this combination was to synergize the hypnotic effects of the barbiturates with the anxiolytic and antiemetic properties of hydroxyzine, creating a versatile medication that could efficiently manage various symptoms associated with sleep disturbances.
Secobarbital, a potent barbiturate with a rapid onset, was included to induce quick sleep initiation, while brallobarbital, with its longer-lasting effects, sustained sleep through the night. Hydroxyzine complemented these actions by mitigating anxiety, improving the overall quality of sleep, and reducing the chances of nausea, a potential side effect of barbiturates. This innovative combination aimed to enhance patient compliance by offering a single-dose solution to multifaceted sleep issues.
Administration and Therapeutic Uses
Typically administered as an oral tablet, Vesparax was prescribed for individuals suffering from severe insomnia that didn’t respond well to standard treatments. The standard dosage was carefully calculated to balance efficacy and safety, minimizing the risks associated with barbiturate overuse. Patients were advised to take Vesparax approximately 30 minutes before bedtime, ensuring the timing of peak drug activity corresponded with their sleep schedules.
Effectiveness of Vesparax in treating insomnia was well-documented in the mid-20th century. Clinical reports indicated that patients achieved faster sleep onset and experienced fewer awakenings throughout the night. Anecdotal evidence from that time highlighted the popularity of Vesparax among both clinicians and patients, who appreciated the efficacy of the medication in treating complex sleep disorders with minimal next-day residual effects. One frequently cited case involved an insomniac who, after years of struggling with other treatments, found notable relief with Vesparax under meticulous medical supervision.
However, it should be noted that the use of Vesparax waned as concerns over barbiturate dependency and potential for abuse became more pronounced. Evolving medical guidelines and the introduction of safer alternatives eventually led to the discontinuation of Vesparax in clinical practice, marking the end of an era for this intriguing combination product.
Decline and Legacy of Brallobarbital
The decline in the use of brallobarbital and Vesparax can largely be attributed to a combination of regulatory changes, medical advancements, and the development of newer, safer pharmaceuticals. During the mid-20th century, growing awareness of the potential for abuse and addiction associated with barbiturates led to increased scrutiny by medical professionals and regulatory bodies. Brallobarbital, in particular, faced severe criticism for its narrow therapeutic index, which made it dangerous if not administered with precise dosage. Overdose and dependency risks were significant drawbacks that overshadowed its efficacy in treating insomnia.
Regulatory changes were pivotal in the declining popularity of brallobarbital. In the 1970s, stricter regulations and controls on barbiturate prescriptions were enacted, significantly limiting their availability. This was in response to rising overdose cases and the increasing recognition of their addictive potential. Additionally, the Controlled Substances Act in the United States reclassified many barbiturates, including brallobarbital, under stricter schedules, further curtailing their use.
Parallel to these regulatory shifts, advancements in medical science facilitated the development of newer classes of hypnotic drugs. Benzodiazepines, which emerged in the 1960s and 70s, offered a safer alternative with a broader therapeutic index and lower risk of overdose. Medications like diazepam and lorazepam became preferred choices for insomnia treatment, effectively surpassing barbiturates like brallobarbital in clinical use.
Brallobarbital also suffered due to its adverse effects. Reports of prolonged sedation, cognitive impairment, and high potential for abuse rendered it less favorable compared to emerging medications. Its decline was further precipitated by the development of modern, non-benzodiazepine sleep aids, such as zolpidem, which targeted insomnia with improved safety profiles and reduced side effects.
Reflecting on the historical significance of brallobarbital, it is evident that while it played a crucial role in the early pharmacological management of insomnia, its legacy is marked by the lessons learned regarding the risks of treatment-related dependency and overdose. Brallobarbital’s history underscores the importance of ongoing pharmaceutical innovation and rigorous regulatory oversight in the development of safe and effective therapeutic agents. As modern medicine continues to evolve, the cautionary tale of brallobarbital remains a valuable reference in the pursuit of optimal insomnia treatments.




Reviews
There are no reviews yet.